Why Is Fluorine More Electronegative Than Chlorine
Fluorine is more electronegative than chlorine therefore it can attract a share pair of electron more easily and strongly than chlorine. The larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is.
What Would Cause An Atom To Have A Low Electronegativity Value Socratic
Thus it can easily accept the.

. Answer 1 of 7. B State whether C is more reactive or. The electron gained also feels a great amount of repulsion from the electrons.
Therefore fluorine more electronegative than chlorine. As we know thatchlorine atom has more electrons and protons than florine so chlorine has to be more electro negative than of florine but it is wrong because florine has 2 shell ie. Hydrogen 407 201 2.
Since halogens are more electronegative than. As we go down a group the atomic radii of the atom increases even though the nuclear charge also increases the atomic radii increases to a greater extent which practically. The electron cloud of F is more dense than that of Cl because Cl is larger in size.
Though fluorine being more electronegative because of its small size there ocurrs repulsions between the added electrons and the already existing 2p electrons. See full answer below. Fluorine though higher than chlorine in the periodic table has a very small atomic size.
A The fluorine atom is smaller than the chlorine atom and there is less shielding from other shells of electrons. This is because the valencebonding electrons are closer to the nucleus in Fluorine than they are Chlorine and others and thus more strongly attracted. The closer it gets to being full we know that electronegativity increases.
F has greater tendency than Cl to attract the shared pair of electrons of a covalent bond. Though fluorine is the most electronegative atom it has lower electron affinity than chlorineIt is due to their compact atomic size which propels it high electron density inside the atomSo the. This is because the electrons in the outermost shell of a fluorine atom are closer together.
Why does fluorine have a higher electronegativity than carbon. B The fluoride ion is smaller than the chloride ion giving it a larger charge density. With Fluorine only needing one more electron to be complete.
The electron affinity of the fluorine is less than chlorine because the size of fluorine is too small as size decreases from left to right inside period whereas chlorine has a larger size to. This makes the fluoride anion so formed unstable highly reactive due to a very high. Since the flourines are more electronegative than the chlorine F is higher in the VIIA column than Cl they will get the bigger share of the electrons and Cl less.
In case of chlorine the size. So electrons cant enter in Florines valence shell because of electrostatic force of rapultion of. KL where as chlorine has 3 shell ie.
Fluorine is more electronegative than chlorine because fluorine is smaller and has its electrons closer to the positively charged nucleus. Thus the bonding pair of electrons are more attracted to the positive nucleus. Why is fluorine more electronegative than carbon.
A qualitative answer is that fluorine is more electronegative than chlorine because the outer electrons in fluorine are less screened from their attraction to the positive nucleus because there. Fluorine is more electronegative than chlorine even then p-flurobenzoic acid is weaker acid than p-chlorobenzoic acid explain. Thus it is more strongly attracted to the delta positive hydrogens on a water molecule.
Allowing for the shielding effect of the 1s electrons the. The big battle for second place is.

Fluorine Is More Electronegative Than Chlorine Is The Statement True Youtube

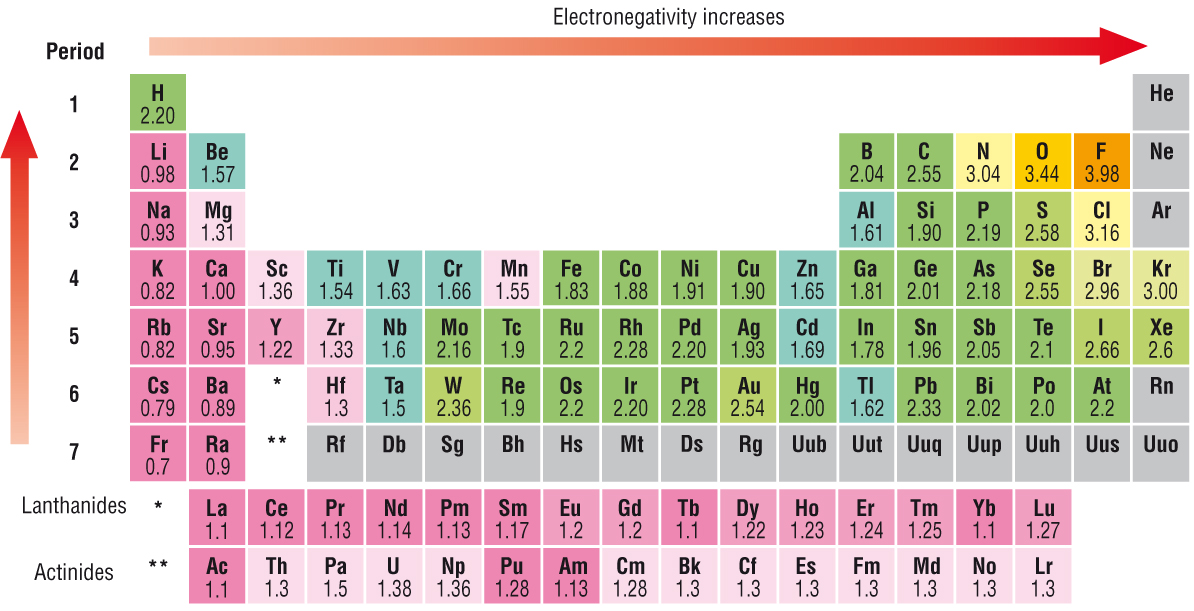
What Is Electronegativity Trends Chart Periodic Table Chemtalk
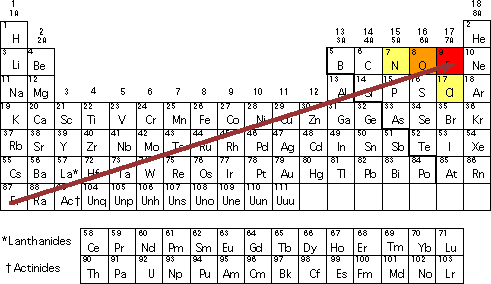
0 Response to "Why Is Fluorine More Electronegative Than Chlorine"
Post a Comment